-
PDF
- Split View
-
Views
-
Cite
Cite
Takumi Takeuchi, Zev Rosenwaks, Gianpiero D. Palermo, A successful model to assess embryo development after transplantation of prophase nuclei, Human Reproduction, Volume 19, Issue 4, 1 April 2004, Pages 975–981, https://doi.org/10.1093/humrep/deh149
Close - Share Icon Share
Abstract
BACKGROUND: Germinal vesicle transplantation (GVT) provides a means of investigating interactions between karyoplasts and cytoplasts isolated from different cells. Technically, GVT can be accomplished with a high degree of efficiency without compromising the maturation of either the human or mouse oocyte nucleus. Although maturation, fertilization and preimplantation development have been established using GVT, full‐term development has been reported only after supplementation with fresh mature ooplasm. In this study, we assess the ability of immature oocytes collected from gonadotrophin‐primed ovaries to mature in vitro after GVT and develop to full‐term. METHODS: GV oocytes were retrieved from either non‐stimulated or pregnant mare’s serum gonadotrophin (PMSG)‐primed female mice. Microsurgically isolated GV karyoplasts were transplanted into previously enucleated oocytes. Oocytes successfully reconstituted by electrofusion were cultured for 14 h to allow nuclear maturation. Metaphase II oocytes were subjected to Piezo‐ICSI, and those fertilized normally were cultured to the blastocyst stage. Some such embryos were transferred to pseudopregnant female mice to examine their potential for normal development. Cumulus‐denuded non‐manipulated oocytes that were matured in vitro served as controls. RESULTS: The reconstitution and maturation rates were comparable in oocytes isolated from PMSG‐primed and from unstimulated ovaries. The rate of normal fertilization in oocytes from primed ovaries was significantly higher than that of their non‐primed counterparts (63.5 versus 39.6%; P < 0.01). This difference was also confirmed in terms of blastocyst development (31.8 versus 7.9%; P < 0.01). Of a total of 70 embryos transferred to the oviduct of five recipient mice, 21.4% developed to normal live offspring. All developed as normal adults and proved to be fertile. The live birth rate was comparable to that obtained using non‐manipulated control oocytes (22.3%). CONCLUSIONS: Higher rates of fertilization and blastocyst formation were obtained after GVT of mouse oocytes isolated from PMSG‐primed ovaries compared with their non‐primed counterparts. These represent the first mouse offspring derived from in vitro matured, cumulus‐denuded oocytes treated by allo‐GVT and fertilized by ICSI. Thus, GVT appears not to impair oocyte maturation, fertilization and pre‐ and post‐implantation development and, after gonadotrophin priming, allows generation of healthy mouse offspring without mature ooplasm supplementation.
Introduction
Oocyte aneuploidy is considered to be responsible for a lower implantation rate and increased pregnancy loss in women of advanced reproductive age. Currently, these women can be successfully treated by oocyte donation, but most couples express interest in utilizing their own gametes. Current clinical practice involves oocyte/embryo selection by preimplantation genetic diagnosis. This procedure, however, can only select among the available conceptuses. In women ≥40 years old, the higher incidence of aneuploidy would leave only a minimal number of normal embryos for replacement in such cases.
Aneuploidy in aged oocytes occurs at a specific maturational step, first meiosis, and is due to non‐disjunction. This results from a dysfunction of the ooplasm which is unable to provide the molecular structural components necessary to generate a normal meiotic spindle. Specific mechanisms that underlie this may be related to mitochondrial DNA mutations (Keefe et al., 1995; Barritt et al., 2000; Schon et al., 2000), oxidative stress (Tarín, 1995) or abnormalities in the ovarian microcirculation (Gaulden, 1992; Van Blerkom et al., 1995; Van Blerkom, 1996). All of these age‐related aberrations can lead to impaired mitochondrial metabolism and so incomplete chromosomal segregation. In fact, a lower mitochondrial membrane potential is associated with an age‐related higher incidence of meiotic spindle abnormalities during oocyte maturation (Battaglia et al., 1996; Volarcik et al., 1998; Wilding et al., 2003).
Germinal vesicle transplantation (GVT) has been proposed as a treatment for correction of age‐related oocyte aneuploidy (Zhang et al., 1999). A putatively normal GV nucleus transplanted into a younger ooplast would have the right molecular and structural components to build a normal meiotic spindle, allowing proper segregation of chromosomes between the constructed oocyte and the first polar body (PB) (Tsai et al., 2000). It remains difficult to prove the feasibility of this concept, however, due to the absence of animal models that simulate human oocyte aneuploidy. Thus far, in only a small number of human oocytes it has been possible to prove that placement of an aged GV nucleus into a younger ooplasm reduces occurrence of aneuploidy (Zhang et al., 1999; Takeuchi et al., 2001; Palermo et al., 2002).
In the hope of establishing such a model in which to test this idea, we have induced mitochondrial damage in GV oocytes and attempted to rescue them by replacing the ooplasm through GVT (Palermo et al., 2002). The results demonstrate that the GVT oocytes can overcome maturational arrest and undergo first meiotic division with normal chromosomal segregation.
A further step to be assessed is the ability of the GVT oocytes to undergo fertilization and normal development (Li et al., 2001; Takeuchi et al., 2001). Although micromanipulation of oocytes requires cumulus cell removal, granulosa cells are crucial for oocyte maturation and for a successful completion of meiosis and full‐term development (Niwa et al., 1976; Shcroeder and Eppig, 1984; Vanderhyden and Armstrong, 1989; Flood et al., 1990; Cecconi et al., 1996). To avoid this problem, a sequential cytoplasmic transfer supplying in vivo matured cytoplasm either at metaphase II (MII) stage (Kono et al., 1996; Bao et al., 2000; Liu et al., 2003) or at pronuclear stage (Liu et al., 2003) was considered necessary.
In some cases, mammalian GV oocytes are able to undergo spontaneous in vitro maturation once isolated from unstimulated ovaries (Pincus and Enzmann, 1935; Edwards, 1965), and this has become the source of immature oocytes for GVT (Takeuchi et al., 1999). However, although they mature at a standard rate, the resulting MII oocytes display an oolemma the fragility of which renders ICSI extremely challenging (Liu et al., 2000; Palermo et al., 2002). Despite of this handicap we have been able to obtain a fertilization rate of up to ∼40%, but the very low number developing a blastocyst (Takeuchi et al., 2002) discouraged us from assessing the potential of these non‐primed oocytes for normal development.
It has been shown that prior gonadotrophin stimulation enhances the ability of oocytes matured in vitro to develop into normal embryos (Schroeder and Eppig, 1989), and the presence of FSH in the medium is known to promote the development of mouse GV oocytes (Merriman et al., 1998; Anderiesz et al., 2000). However, because these studies utilized cumulus‐invested oocytes, there is no definitive information as to the effect of gonadotropin‐priming on fertilizability or development of cumulus‐denuded GVT oocytes, nor whether normal offspring can be obtained directly from GVT oocytes without cytoplasmic supplementation (Kono et al., 1996; Bao et al., 2000).
In this study, we assessed the effect on future development of gonadotrophin‐priming prior to collection of GV oocytes. In order to assess their ability to undergo full‐term development after spontaneous maturation, oocytes transplanted with GV nuclei were subjected to ICSI and transferred to pseudopregnant female mice, with non‐manipulated GV oocytes matured in vitro after denudation serving as controls. To assess the genetic constitution of the resulting embryos, karyotyping was performed randomly at the 2‐cell stage. The fertility status of the first generation pups was also assessed.
Materials and methods
Animals
The experimental protocol was fully approved by the Institutional Animal Care and Use Committee of Cornell University Medical College. B6D2F1 (Jackson Laboratory, Bar Harbor, ME, USA) and CD‐1 (Charles River Laboratory, Wilmington, MA, USA) mice were purchased and housed (The Research Animal Resource Center of Weill Medical College of Cornell University) in a temperature‐ and light‐controlled room on a 14 h light:10 h dark photoperiod and were provided with food and water ad libitum.
Gamete collection
Full‐grown GV oocytes of ≥75 µm diameter (Bao et al., 2000) from 7–11 week B6D2F1 female mice were retrieved either from non‐stimulated ovaries or those treated by pregnant mare’s serum gonadotrophin (PMSG; Sigma, St Louis, MO, USA) 48 h prior to the procedure. Cumulus cells were removed by repeated aspiration through a hand‐drawn pipette. In order to prevent spontaneous germinal vesicle breakdown, oocytes were cultured in Waymouth’s medium (MB752/1; Invitrogen, Carlsbad, CA, USA) supplemented with a phosphodiesterase inhibitor (0.2 mmol/l 3‐isobutyl‐1‐methylxanthine; IBMX; Sigma), 0.23 µmol/l pyruvate (Sigma) and 5% fetal bovine serum (Invitrogen) until the GVT procedure. Mature MII oviductal oocytes were collected from the same mouse strain after PMSG and HCG treatment as described previously (Takeuchi et al., 1999). After cumulus removal, MII oocytes were incubated in a CZB medium (Chatot et al., 1990) until ICSI. Spermatozoa were obtained from caudae epididymides of mature male mice of the same strain and kept in CZB medium for at least 1 h, prior to injection.
Nuclear transplantation and oocyte maturation in vitro
All the micromanipulation and electrofusion procedures were performed under an inverted microscope equipped with a hydraulic micomanipulator as described previously (Takeuchi et al., 1999). Enucleation was conducted in a medium supplemented with 25 µg/ml of cytochalasin B (Sigma) and was performed with a 25 µm glass cylindrical pipette after making a slit in the zona pellucida with a microneedle. Isolated GV karyoplasts were transferred into enucleated oocytes of the same maturational stage. Each grafted oocyte was manually aligned between two microelectrodes, and cell fusion was subsequently induced by direct electrical current (Takeuchi et al., 1999).
Oocytes reconstituted successfully were rinsed and cultured in IBMX‐free Waymouth’s medium for 14 h to allow nuclear maturation. Oocyte maturation was judged according to the appearance of a single PB. Non‐manipulated cumulus‐free GV oocytes served as controls.
Piezo‐ICSI and fertilization assessment
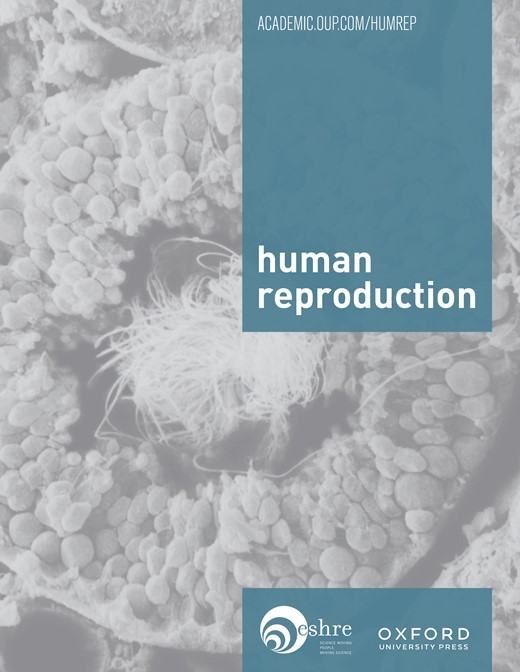
The reconstituted oocytes after in vitro maturation were injected with a single dissected sperm head by the aid of a Piezo actuator (PiezoDrill; Burleigh Instruments, Inc., Fishers, NY, USA and PMM‐150FU, PrimeTech, Ibaragi, Japan), essentially as reported previously (Wakayama and Yanagimachi, 1998). Briefly, a single spermatozoon was aspirated into an injection pipette of ∼5 µm inner diameter. The sperm head and tail were separated by applying a single or a few piezo pulses to the neck region, then the head alone was injected. Approximately 5–6 h after the injection, the oocytes surviving injection were evaluated for the presence of two distinct pronuclei (PN) and a clear second PB, the criteria of normal fertilization (Figure 1). Oocytes with an extruded second PB and the presence of at least one PN were designated as activated oocytes.
Cytogenetic analysis
Some 2‐cell embryos were cultured overnight in potassium simplex optimized medium with amino acids (KSOMAA; Cell & Molecular Technologies, Phillipsburg, NJ, USA) containing 0.25 µg/ml of demecolcine (Sigma) to synchronize metaphase nuclei, and then processed for chromosome analysis by gradual fixation (Takeuchi et al., 1999). Chromosome spreads were stained with Giemsa.
Pre‐ and post‐implantation development of nuclear transplanted oocytes after ICSI
Fertilized oocytes were incubated further in KSOMAA medium for up to 96 h in order to assess embryonic cleavage compared with that of control oocytes matured in vitro.
Some experimental embryos at the 2‐cell stage were surgically transferred into oviducts of CD‐1 foster mothers that had been mated with a vasectomized male of the same strain during the previous night. Because the initial part of this study revealed a significantly impaired blastocyst formation rate in PMSG non‐primed oocytes, only embryos derived from stimulated ovaries were utilized for this experiment. Some 2‐cell stage embryos derived from oocytes matured in vivo, or those matured in vitro without GVT, were transferred to pseudopregnant females as controls.
Data analysis
The χ2‐test was utilized to identify differences in cell survival, maturation, fertilization, blastocyst formation and live birth rates observed between the different groups. Significance was set at P = 0.05. All statistical computations were conducted using StatView (SAS Institute, Inc., Cary, NC, USA). Only significant differences are noted in the text and/or tables.
Results
Effect of PMSG priming on GVT and maturation
After GVT, 83.9% of 347 intact GV oocytes isolated from PMSG‐stimulated ovaries survived and successfully reconstituted, a rate comparable to that of unprimed oocytes (79.5% of 195), with the in vitro maturation rate being the same in both groups (89.3 versus 89.7%).
Effect of PMSG priming on fertilization and embryonic cleavage after ICSI
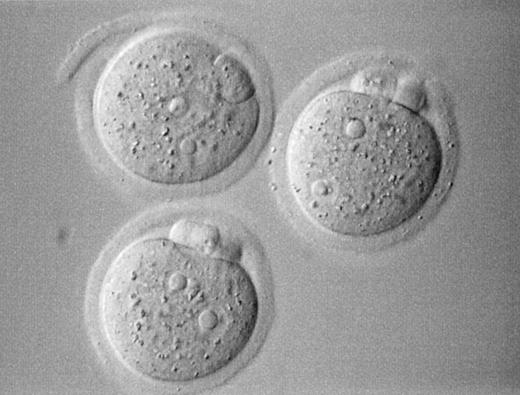
Fertilization characteristics and embryonic cleavage of GVT oocytes are summarized in Table I. While cell survival (74.0 versus 80.8%) and oocyte activation per surviving oocyte (92.9 versus 95.2%) were not different between the unprimed and stimulated oocytes, respectively, more oocytes from PMS stimulated ovaries were fertilized normally (63.5 versus 39.6%; P < 0.01). A beneficial effect of priming was also confirmed for the rate of blastocyst formation (31.8% versus 7.9; P < 0.01) (Figure 2). A fertilization rate of 64.5% (40 out of 62) and a blastocyst formation rate of 35.0% (14 out of 40) observed in non‐manipulated in vitro matured control oocytes obtained from PMSG‐stimulated ovaries were not different from those of PMSG‐primed GVT oocytes.
Cytogenetics of the fertilized oocytes
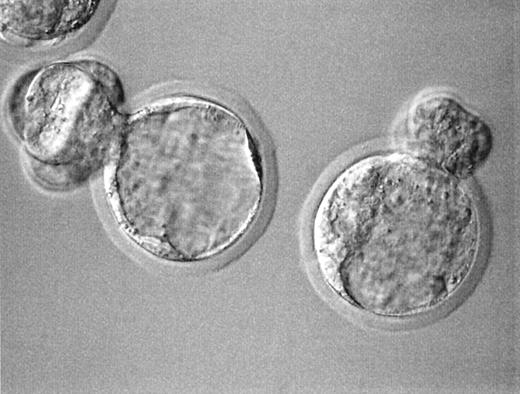
Sixteen 2‐cell stage embryos were processed, so that 32 metaphase nuclei were fixed for a cytogenetic assessment. Of the 17 (53.1%) analysable blastomeres, 14 (82.4%) had a normal diploid set of chromosomes (Figure 3).
Post‐implantation development of the GVT oocytes
When a total of 70 2‐cell embryos derived from GVT oocytes were transferred to five recipients, 15 live offspring were born on day 20. This live birth rate (range 11.1–35.0%) was comparable to that of control non‐manipulated GV oocytes (range 11.8–41.7%) (Table II). All the offspring developed as adults without any apparent abnormalities (Figure 4). The coat colour of offspring was black (n = 7), grey (n = 5) or brown (n = 3) as expected. These young mice proved to be fertile after puberty, with normal litter sizes (eight to 10). A total of 17 live offspring (37.8%) was obtained after oviductal transfer of 45 2‐cell embryos derived from in vivo matured oocytes. The live birth rate per transferred embryo was not significantly different between the three groups.
Discussion
We have shown that oocytes reconstituted by GVT can complete the first meiotic division in vitro at a rate comparable to that of control oocytes, and that the technique itself did not increase the frequency of abnormal segregation of chromosomes. In line with a previous report (Takeuchi et al., 1999), this confirms that GVT can be accomplished with a high efficiency, and without impairing subsequent oocyte maturation. Moreover, mouse oocytes reconstituted by GVT can be fertilized normally by ICSI and are able to undergo embryonic cleavage, as observed in other species (Li et al., 2001; Takeuchi et al., 2001; Palermo et al., 2002). Although the nuclear maturation rate was comparable in oocytes from primed and non‐primed females, gonadotrophin treatment significantly enhanced the fertilization and embryonic cleavage rates. After replacement into the oviducts of pseudopregnant female mice, full‐term development of some GVT oocytes was achieved at a rate comparable to that of non‐manipulated cumulus cell‐deprived oocytes matured in vitro. Previously, full‐term mouse offspring were generated from GVT oocytes only through a complex sequential addition of mature ooplasm (Kono et al., 1996; Bao et al., 2000; Liu et al., 2003). Here, however, mouse GVT oocytes developed to term without the need for supplementation with mature ooplasm. The present success depended on bypassing normal steps leading to fusion of gametes and on gonadotrophin priming to synchronize follicular development.
Mouse offspring can be obtained from in vitro matured oocytes more readily by ICSI than by conventional in vitro insemination, demonstrating the ability of direct injection to bypass the eventual changes in the zona pellucida and oolemma during in vitro maturation (Yamazaki et al., 2001). Thus, in accordance with the high fertilization rates of in vitro matured oocytes isolated from preantral follicles (Liu et al., 2002), ICSI appears to be the best way to fertilize oocytes matured in vitro, particularly when deprived of their cumulus cells.
With ICSI we were able to obtain a consistent fertilization rate of 65%, compared with the previous 35% with in vitro matured denuded oocytes after standard insemination (Schroeder and Eppig, 1984). Only when cumulus cells were present did the fertilization rate reach 73% in in vitro matured oocytes (Schroeder and Eppig, 1984), indicating cumulus cell‐induced modifications of the sperm membrane that in our GVT oocytes are obviously missing but are successfully bypassed by ICSI. In the present study, >90% of the oocytes surviving the ICSI procedure showed signs of activation regardless of gonadotrophin priming. The large majority had a normal fertilization rate, while the remainder displayed a single PN. The relatively higher incidence of abnormal fertilization was due to a failure of the sperm nucleus to decondense in the still immature cytoplasm (Lee et al., 2003). Oocytes matured in vitro may lack cytoplasmic factors required for male PN formation, such as glutathione (Funahashi et al., 1995) or ATP (Collas and Poccia, 1998), which are found in MII but not GV ooplasm (Maeda et al., 2000). It is possible that a subgroup matured in vitro had reduced levels of ‘sperm decondensing factor(s)’ due to suboptimal maturation. Therefore, it is likely that despite the obvious nuclear maturity, oocytes may not yet complete maturation of their cytoplasm, and exhibit an asynchronous maturation between nucleus and cytoplasm, with consequent abnormal sperm nuclear decondensation.
Here, we have demonstrated that gonadotrophin stimulation enhances fertilization after ICSI and later cleavage of GVT oocytes. Once granulosa cells of preantral follicles experience the stimulations of FSH and develop estrogen responsiveness, many proliferate rapidly, so ensuring the formation of the preovulatory follicle (Rao et al., 1978). Both the proliferation and differentiation of granulosa cells are facilitated by the increasing intracellular level of cyclin D2 under FSH regulation (Robker and Richards, 1998). Appropriate proliferation and terminal differentiation of granulosa cells are critical for normal folliculogenesis and, therefore, for organized oogenesis.
It is clear that FSH priming can ‘rescue’ a cohort of small antral follicles destined to undergo atresia during the follicular phase of a natural cycle (Schramm and Bavister, 1994), probably through inhibition of granulosa cell apoptosis (Braw and Tsafriri, 1980; Hsueh et al., 1994). Gonadotrophins may do so by regulating ovarian glutathione (GSH) synthesis by modulating glutamate cystein ligase subunit expression (Luderer et al., 2001), since a GSH precursor, N‐acetyl cystein, inhibits apoptosis as effectively as the FSH in cultured antral follicles (Tilly and Tilly, 1995), and high concentrations of GSH in mature oocytes are critical for fertilization and early embryo development (Calvin et al., 1986; Perreault et al., 1988; Gardiner and Reed, 1995). Thus gonadotropin priming enhances overall oocyte development simply by recruiting more synchronous non‐apoptotic oocytes through GSH synthesis. Although in a recent report it has been shown that FSH priming has no beneficial effect on in vitro maturation of human oocytes (Lin et al., 2003), they were cultured with an intact cumulus oophorus, exposed to HCG in vivo 36 h prior to retrieval, and were obtained from women with polycystic ovaries. Thus, culture conditions of the oocytes in the above work were different from those of the current study. Therefore, the beneficial effects of gonadotropin stimulation on the developmental competence of mouse oocytes may become more pronounced when maturation occurs in the absence of cumulus cells.
Although the replacement with younger cytoplasm appeares to be a promising approach to correction of age‐related aneuploidy (Zhang et al., 1999; Takeuchi et al., 2001; Palermo et al., 2002), this remains to be proven. The cumbersome procedure in the mouse, a species known for its difficulty in surviving the ICSI procedure, together with the shortage of spare human oocytes have contributed to the ‘premature’ decision to offer the procedure to couples suffering from age‐related infertility (J.Grifo, ASRM Annual Meeting 1998). This approach, together with a less invasive procedure called ‘cytoplasmic transfer’ (Barritt et al., 2001), has prompted the Food and Drug Administration intervention and consequent regulations (Zoon, 2001).
The ability to generate offspring from immature mouse oocytes in a direct manner provides a feasible model in which to study nuclear transplantation and to examine whether this may offer a way of correcting oocyte aneuploidy. In addition, the occurrence of full‐term development without additional cytoplasmic infusions avoids the further complication of foreign mtDNA heteroplasmy (Cummins, 2001; 2002) and its transmission to future generations (Van Blerkom et al., 1998; Barritt et al., 2001; St John, 2002). Furthermore, the epigenetics of the reconstituted oocytes and derived conceptuses should be investigated, since altered DNA methylation patterns have been observed in offspring produced by nuclear transplantation (Reik et al., 1993; 2001; Humpherys et al., 2001; Hawes et al., 2002). A final step to assess the efficacy of GVT in prevention of oocyte aneuploidy would be reproduction of a system that simulates the damage present in oocytes isolated from older women. This may be, for example, accomplished by specifically altering membrane potentials of mitochondria (Palermo et al., 2002; Takeuchi et al., 2003).
In conclusion, we demonstrate that mouse GVT oocytes can undergo in vitro maturation, fertilization and full‐term development without supplementation with mature ooplasm. Oocyte survival after ICSI, fertilization and embryo development did not differ from that observed in non‐manipulated, in vitro matured oocytes.
Acknowledgements
We are very appreciative to Professor J.Michael Bedford for his critical reading and to Ms Queenie V.Neri for editing the manuscript.
Figure 1. Mouse zygotes derived from germinal vesicle transplanted oocytes 6 h following ICSI (original magnification 400×).
Figure 2. Blastocysts developed from germinal vesicle transplanted (GVT) oocytes 96 h after ICSI. Note hatching from the slit on the zona made during GVT (original magnification 400×).
Figure 3. Metaphase diploid chromosomes observed in a blastomere isolated from a 2‐cell stage embryo (original magnification 600×).
Figure 4. The foster mother (white) and four young (black) derived from germinal vesicle transplanted oocytes.
ICSI fertilization and embryonic cleavage of PMSG‐primed and non‐primed oocytes after germinal vesicle transplantation
| Origin of oocytes [n (%)] | ||
| Non‐primed | PMSG‐primed | |
| (a) ICSI inseminated oocytes | 96 | 104 |
| (b) Survived oocytes (% of a) | 71 (74.0) | 84 (80.8) |
| (c) Activated oocytes (% of a) | 66 (68.7) | 80 (76.9) |
| (d) Fertilized oocytes (% of a) | 38 (39.6)a | 66 (63.5)a |
| (e) 2‐cell stage embryos (% of d) | 35 (92.1) | 63 (95.5) |
| (f) Blastocysts (% of d) | 3 (7.9)b | 21 (31.8)b |
| Origin of oocytes [n (%)] | ||
| Non‐primed | PMSG‐primed | |
| (a) ICSI inseminated oocytes | 96 | 104 |
| (b) Survived oocytes (% of a) | 71 (74.0) | 84 (80.8) |
| (c) Activated oocytes (% of a) | 66 (68.7) | 80 (76.9) |
| (d) Fertilized oocytes (% of a) | 38 (39.6)a | 66 (63.5)a |
| (e) 2‐cell stage embryos (% of d) | 35 (92.1) | 63 (95.5) |
| (f) Blastocysts (% of d) | 3 (7.9)b | 21 (31.8)b |
aχ2, 2 × 2, 1 df, Effect of PMSG priming on fertilization rate, P < 0.01.
bχ2, 2 × 2, 1 df, Effect of PMSG priming on blastocyst formation rates, P < 0.01.
PMSG = pregnant mare’s serum gonadotrophin.
ICSI fertilization and embryonic cleavage of PMSG‐primed and non‐primed oocytes after germinal vesicle transplantation
| Origin of oocytes [n (%)] | ||
| Non‐primed | PMSG‐primed | |
| (a) ICSI inseminated oocytes | 96 | 104 |
| (b) Survived oocytes (% of a) | 71 (74.0) | 84 (80.8) |
| (c) Activated oocytes (% of a) | 66 (68.7) | 80 (76.9) |
| (d) Fertilized oocytes (% of a) | 38 (39.6)a | 66 (63.5)a |
| (e) 2‐cell stage embryos (% of d) | 35 (92.1) | 63 (95.5) |
| (f) Blastocysts (% of d) | 3 (7.9)b | 21 (31.8)b |
| Origin of oocytes [n (%)] | ||
| Non‐primed | PMSG‐primed | |
| (a) ICSI inseminated oocytes | 96 | 104 |
| (b) Survived oocytes (% of a) | 71 (74.0) | 84 (80.8) |
| (c) Activated oocytes (% of a) | 66 (68.7) | 80 (76.9) |
| (d) Fertilized oocytes (% of a) | 38 (39.6)a | 66 (63.5)a |
| (e) 2‐cell stage embryos (% of d) | 35 (92.1) | 63 (95.5) |
| (f) Blastocysts (% of d) | 3 (7.9)b | 21 (31.8)b |
aχ2, 2 × 2, 1 df, Effect of PMSG priming on fertilization rate, P < 0.01.
bχ2, 2 × 2, 1 df, Effect of PMSG priming on blastocyst formation rates, P < 0.01.
PMSG = pregnant mare’s serum gonadotrophin.
Full‐term development of embryos derived from GV transplanted and intact oocytes
| Origin of oocytes | ||
| Non‐manipulated | GV transplanted | |
| ICSI inseminated oocytes (n) | 176 | 122 |
| Fertilized oocytes [n (%)] | 103 (58.5) | 80 (65.6) |
| Replaced embryos (mean ± SD) | 94 (15.7 ± 2) | 70 (14.0 ± 5) |
| Recipients (n) | 6 | 5 |
| Live offspring [n (%)] | 21 (22.3) | 15 (21.4) |
| Origin of oocytes | ||
| Non‐manipulated | GV transplanted | |
| ICSI inseminated oocytes (n) | 176 | 122 |
| Fertilized oocytes [n (%)] | 103 (58.5) | 80 (65.6) |
| Replaced embryos (mean ± SD) | 94 (15.7 ± 2) | 70 (14.0 ± 5) |
| Recipients (n) | 6 | 5 |
| Live offspring [n (%)] | 21 (22.3) | 15 (21.4) |
GV = germinal vesicle.
Full‐term development of embryos derived from GV transplanted and intact oocytes
| Origin of oocytes | ||
| Non‐manipulated | GV transplanted | |
| ICSI inseminated oocytes (n) | 176 | 122 |
| Fertilized oocytes [n (%)] | 103 (58.5) | 80 (65.6) |
| Replaced embryos (mean ± SD) | 94 (15.7 ± 2) | 70 (14.0 ± 5) |
| Recipients (n) | 6 | 5 |
| Live offspring [n (%)] | 21 (22.3) | 15 (21.4) |
| Origin of oocytes | ||
| Non‐manipulated | GV transplanted | |
| ICSI inseminated oocytes (n) | 176 | 122 |
| Fertilized oocytes [n (%)] | 103 (58.5) | 80 (65.6) |
| Replaced embryos (mean ± SD) | 94 (15.7 ± 2) | 70 (14.0 ± 5) |
| Recipients (n) | 6 | 5 |
| Live offspring [n (%)] | 21 (22.3) | 15 (21.4) |
GV = germinal vesicle.
References
Anderiesz C, Ferraretti AP, Magli S, Fiorentino A, Fortini D, Gianaroli L, Jones GM and Trounson AO (
Bao S, Obata Y, Carroll J, Domeki I and Kono T (
Barritt JA, Cohen J and Brenner CA (
Barritt JA, Brenner CA, Malter HE and Cohen J (
Battaglia DE, Goodwin P, Klein NA and Soules MR (
Braw RH and Tsafriri A (
Calvin HI, Grosshans K and Blake EJ (
Cecconi S, D’Aurizio R and Colonna R (
Chatot CL, Torres I and Ziomek CA (
Collas P and Poccia D (
Cummins JM (
Cummins JM (
Edwards RG (
Flood JT, Chillic CF, Van Uem JF, Iritani A and Hodgen GD (
Funahashi H, Stumpf TT, Cantley TC, Kim NH and Day BN (
Gardiner CS and Reed DJ (
Gaulden ME (
Hawes SM, Sapienza C and Latham KE (
Hsueh AJW, Billig H and Tsafriri A (
Humpherys D, Eggan K, Akutsu H, Hochedlinger K, Rideout WM 3rd, Biniszkiewicz D, Yanagimachi R and Jaenisch R (
Keefe DL, Niven‐Fairchild T, Powell S and Buradagunta S (
Kono T, Obata Y, Yoshimzu T, Nakahara T and Carroll J (
Lee JW, Tian XC and Yang X (
Li GP, Chen DY, Lian L, Sun QY, Wang MK, Liu JL, Li JS and Han ZM (
Lin YH, Hwang JL, Huang LW, Mu SC, Seow KM, Chung J, Hsieh BC, Huang SC, Chen CY and Chen PH (
Liu H, Zhang J, Krey LC and Grifo JA (
Liu H, Chang HC, Zhang J, Grifo J and Krey LC (
Liu J, Rybouchkin A, Van der Elst J and Dhont M (
Luderer U, Kavanagh TJ, White CC and Faustman EM (
Maeda Y, Yanagimachi H, Tateno H, Usui N and Yanagimachi R (
Merriman JA, Whittingham DG and Carroll J (
Niwa K, Miyake M, Iritani A and Nishikawa Y (
Palermo GD, Takeuchi T and Rosenwaks Z (
Perreault SD, Barber RR and Slott VL (
Pincus G and Enzmann EV (
Rao MC, Midgley AR Jr and Richards JS (
Reik W, Romer I, Barton SC, Surani MA, Howlett SK and Klose J (
Reik W, Dean W and Walter J (
Robker RL and Richards JS (
Schon EA, Kim SH, Ferreira JC, Magalhaes P, Grace M, Warburton D and Gross SJ (
Schramm RD and Bavister BD (
Schroeder AC and Eppig JJ (
Schroeder AC and Eppig JJ (
St John JC (
Takeuchi T, Ergün B, Huang TH, Rosenwaks Z and Palermo GD (
Takeuchi T, Gong J, Veeck LL, Rosenwaks Z and Palermo GD (
Takeuchi T, Rosenwaks Z and Palermo GD (
Takeuchi T, Rosenwaks Z and Palermo GD (
Tarín JJ (
Tilly JL and Tilly KI (
Tsai MC, Takeuchi T, Bedford JM, Reis MM, Rosenwaks Z and Palermo GD (
Van Blerkom J (
Van Blerkom J, Davis P and Lee J (
Van Blerkom J, Sinclair J and Davis P (
Vanderhyden BC and Armstrong DT (
Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul‐Karim FW and Hunt P (
Wakayama T and Yanagimachi R (
Wilding M, De Placido G, De Matto L, Marino M, Alviggi C and Dale B (
Yamazaki Y, Wakayama T and Yanagimachi R (
Zhang J, Wang CW, Krey L, Liu H, Meng L, Blaszczyk A, Adler A and Grifo J (
Zoon KC (