-
PDF
- Split View
-
Views
-
Cite
Cite
David Møbjerg Kristensen, Ulla Hass, Laurianne Lesné, Grete Lottrup, Pernille Rosenskjold Jacobsen, Christele Desdoits-Lethimonier, Julie Boberg, Jørgen Holm Petersen, Jorma Toppari, Tina Kold Jensen, Søren Brunak, Niels E. Skakkebæk, Christine Nellemann, Katharina M. Main, Bernard Jégou, Henrik Leffers, Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat, Human Reproduction, Volume 26, Issue 1, January 2011, Pages 235–244, https://doi.org/10.1093/humrep/deq323
Close - Share Icon Share
Abstract
More than half of pregnant women in the Western world report intake of mild analgesics, and some of these drugs have been associated with anti-androgenic effects in animal experiments. Intrauterine exposure to anti-androgens is suspected to contribute to the recent increase in male reproductive problems, and many of the anti-androgenic compounds are like the mild analgesics potent inhibitors of prostaglandin synthesis. Therefore, it appears imperative to further investigate the potential endocrine disrupting properties of mild analgesics.
In a prospective birth cohort study, 2297 Danish and Finnish pregnant women completed a questionnaire and 491 of the Danish mothers participated in a telephone interview, reporting on their use of mild analgesics during pregnancy. The testicular position of newborns was assessed by trained paediatricians. In rats, the impact of mild analgesics on anogenital distance (AGD) after intrauterine exposure was examined together with the effect on ex vivo gestational day 14.5 testes.
In the Danish birth cohort, the use of mild analgesics was dose-dependently associated with congenital cryptorchidism. In particular, use during the second trimester increased the risk. This risk was further increased after the simultaneous use of different analgesics. The association was not found in the Finnish birth cohort. Intrauterine exposure of rats to paracetamol led to a reduction in the AGD and mild analgesics accordingly reduced testosterone production in ex vivo fetal rat testes.
There was an association between the timing and the duration of mild analgesic use during pregnancy and the risk of cryptorchidism. These findings were supported by anti-androgenic effects in rat models leading to impaired masculinization. Our results suggest that intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders.
Introduction
Paracetamol (acetaminophen) and non-steroidal anti-inflammatory drugs (NSAIDs) acetylsalicylic acid (aspirin) and ibuprofen are widely used mild analgesics. In both Europe and the USA, more than 50% of pregnant women report intake of mild analgesics with the majority using paracetamol (Werler et al., 2005; Rebordosa et al., 2008b). Studies from the 1980s have suggested a link between prenatal exposure to mild analgesics and reduced masculinization in animals (Gupta and Goldman, 1986; Gupta, 1989). This is of concern, since several reports have indicated an increase in the incidence of male reproductive disorders over the recent decades (Swan et al., 1997; Boisen et al., 2004, 2005; Richiardi et al., 2004) and the geographical distribution together with migration studies (Hemminki and Li, 2002) have suggested that lifestyle and environmental factors play key roles in the pathogenesis.
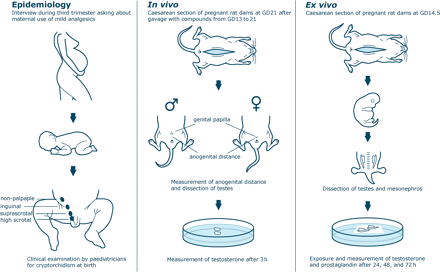
Reproductive disorders such as congenital cryptorchidism, hypospadias, poor semen quality and testicular cancer have been hypothesized to constitute a testicular dysgenesis syndrome (TDS) of fetal origin due to interference in testis development (dysgenesis) (Skakkebaek et al., 2001). Accordingly, data from rat studies have indicated that androgen deficiency during a critical male programming window from gestational day (GD) 15.5–17.5 (corresponding to 8–14 weeks of gestation in humans) leads to cryptorchidism, hypospadias, compromised fertility and reduction in anogenital distance (AGD; Welsh et al., 2008). However, the agent(s) leading to androgen insufficiency and their point of endocrine disruption have remained elusive. We have recently found that many of the endocrine disrupting compounds that reduce masculinization in animal studies by interfering with Leydig cell function and differentiation are potent inhibitors of prostaglandin (PG) synthesis (D. M. Kristensen et al., submitted for publication). This could suggest that these endocrine disrupting compounds damage male reproductive function by inhibiting PG synthesis. This hypothesis is supported by the previous indications that mild analgesics, which relieve pain and reduce inflammation by reducing PG synthesis, may reduce testosterone production (Gupta and Goldman, 1986; Gupta, 1989). If so, pharmaceutical PG inhibitors, at the doses used by humans, could act as endocrine disruptors. In this study, we investigated whether mild analgesics have effects that manifest as male reproductive disorders using a human prospective birth cohort and experimental animal models (Fig. 1).
Schematic representation of the three segments of the study. (i) A prospective human study where pregnant women in the third trimester were specifically asked about their use of mild analgesics. Shortly after birth testicular position was examined by trained paediatricians; (ii) an in vivo/intrauterine study where pregnant rat dams were exposed to the mild analgesics from GD13 to 21 followed by measurement of the fetuses’ AGD and testosterone production; (iii) an ex vivo study where testis from male rat fetuses were incubated for 3 days in media with or without the test compounds. The media concentration of PGD2 and testosterone were measured after 24, 48 and 72 h.
Materials and Methods
Design of the human study
The prospective birth cohort study was conducted at the University Hospital of Copenhagen (Rigshospitalet and Hvidovre Hospital) in Denmark and the Turku University Central Hospital in Finland as described previously (Boisen et al., 2004). Researchers from both countries closely collaborated in study design and recruitment, and examinations were completely standardized. To keep inter-observer variation to a minimum, bi-national workshops were held regularly and borderline cases were examined by two researchers from the national study groups. The study was done according to the Helsinki Declaration and was approved by the local Danish and Finnish ethics committees and the Danish Data Protection Agency. Written informed consent was given by all parents.
Eligible women resident in hospital referral areas were recruited consecutively during pregnancy. To obtain genetically well-defined populations, only families who met the following criteria were included: both parents and grandparents of the unborn child should have been born and raised in Denmark or Finland with a maximum residence abroad of 3 years for the mother and 10 years for the father and grandparents. Gestational age was based on routine ultrasonographical findings in pregnancy week 18–20, if available. In the remaining cases, the last menstrual period was used. Birthweight was obtained from birth records. The examination technique and the definition of cryptorchidism developed by Scorer (1964) were applied. The testis was defined as cryptorchid if it was found high scrotal, supra-scrotal, inguinal or non-palpable after clinical examination by trained paediatricians.
In the Danish cohort, 2521 (22% of all eligible) mothers entered the study and 1071 boys were examined at birth. A total of 5 boys were excluded as dependent cases and 26 were excluded due to missing data. The mothers of the 1040 resulting boys participated in a self-administered written questionnaire in the third trimester (834 boys) or in a computer assisted telephone interview in the third trimester as part of the Danish National Birth Cohort (491 boys) (Olsen et al., 2001), answering questions concerning disease and medicine use during the pregnancy. Mothers of 285 boys participated in both the telephone interview and the questionnaire.
In Finland, 2728 families (24% of all eligible) entered the study and a total of 1499 boys were examined at birth. Of these, 25 boys were excluded as dependent cases as an older brother had already been included in the study and 4 were excluded due to missing data. Of the resulting 1470 boys, 1463 had mothers who participated in a self-administered written questionnaire during the third trimester, answering questions concerning disease and medicine use during the pregnancy.
The written questionnaire in both countries assessed medication in general (‘Have you taken any medication during this pregnancy’), its indication, name, dosage and gestational week of administration, whereas the computer-assisted telephone interview performed only in Denmark specifically addressed the use of analgesics (‘Have you taken any pain-relief medicine during this pregnancy, e.g. normal painkillers or stronger brands?’). If ‘yes', mothers were asked to specify the product and gestational weeks of use.
Animal models
All animal experiments were approved by the local Danish and French ethics committees. Paracetamol and acetylsalicylic acid used in animal experiments were purchased from Sigma-Aldrich (St Louis, MO, USA).
Animal intrauterine studies were conducted after the standardized procedure for detection of anti-androgenic compounds using AGD as readout for the fetal testosterone level and hence masculinization as described previously (Vinggaard et al., 2005). Compounds were administered by gavage from GD13 to 21 and Caesarean section at GD21 using Wistar rats. Paracetamol was administrated in subtoxic doses of 150, 250 and 350 mg/kg/day (Ghanem et al., 2009), whereas acetylsalicylic acid was administered in doses of 150, 200 and 250 mg/kg/day with a lower top dose due to its known adverse effect on pregnancies (Gupta et al., 2003). For dose–response analysis, AGD data were analysed by the calculated AGD index (AGDi), defined as AGD divided by the cube root of the body weight (Gallavan et al., 1999). Analysis of testosterone and testosterone production in GD21 fetal testes were performed as described previously (Vinggaard et al., 2005).
An ex vivo organotypic culture system was used to examined exposure effects at the time of fetal testosterone production initiation at GD14.5. The culture system supports normal differentiation of the Sprague–Dawley rat testes for 3 days (Habert et al., 1991) and has been validated for toxicological studies (Lassurguere et al., 2003; Chauvigne et al., 2009). The experiments were performed as described previously (Chauvigne et al., 2009), however, with two testes in each experiment to increase the amount of secreted PGD2 and testosterone. PGD2 and testosterone were measured with Prostaglandin D2-Mox EIA kit (Cayman Chemicals, Ann Arbor, MI, USA) and Coat-A-Count Total Testosterone (Siemens, Los Angeles, CA, USA), respectively.
Statistics
Statistical analysis of the human data was performed using SPSS 16.0 (Chicago, IL, USA). Differences between the groups were tested with Fisher's exact test. We calculated odds ratios (ORs, 95%) by logistic regression, and the model was adjusted for disease reported during pregnancy (indication to treat), use of other medications during pregnancy, gestational age and twins (only written questionnaires), whereas no confounding effects was observed with birthweight, twins (only the telephone interview), mother's age, smoking, chronic disease and infectious disease.
Animal data were analysed using GraphPad Prism version 4 (La Jolla, CA, USA) and SAS (Cary, NC, USA). Values are expressed as the mean ± SEM. Data from rat intrauterine exposure experiments were analysed using analysis of variance (ANOVA) followed by Dunnett's post hoc test with litter as the statistical unit and included as a random factor in a mixed model. Data for fetal rat testes culture experiments were analysed using a two-sided unpaired Student's t-test.
Results
Prospective birth cohort study
In the self-administrated questionnaire, 26.1% (218 of 834) of Danish mothers reported the use of mild analgesics as opposed to 56.2% (276 of 491) in the computer-assisted telephone interview. Among the 285 Danish mothers who completed both, 30.9% (88 of 285) reported use in the questionnaire as opposed to 57.2% (163 of 285) in the computer-assisted telephone interview. These findings indicated that many mothers did not consider mild analgesics as medication and hence strongly underreported their use unless specifically asked. We therefore only included the data from the computer-assisted telephone interview from the Danish part of the study (for Danish questionnaire results, see Supplementary data, Tables SI and SII). Reasons for using mild analgesics were headache in 66.5%, muscle ache in 6.4% and other types of pain accounted for 8.7%. Fever, inflammation and influenza/cold accounted for 6.9% and several of before mentioned reasons for 6.9%. Other reasons and no reason accounted for the final 4.6%.
Of the mothers with cryptorchid sons, 64.3% (27 of 42) reported the use of mild analgesic during pregnancy versus 55.5% (249 of 449) of mothers with healthy boys [adjusted OR 1.43 (0.73–2.79), P = 0.33; Table I]. The higher prevalence of cryptorchidism became significant for mothers reporting use of more than one specific analgesic [adjusted OR 7.55 (1.94-29.3), P = 0.007].
Maternal use of mild analgesics during pregnancy in relation to congenital cryptorchidism.
| . | Prevalence (%) . | Cryptorchidisma [n (%)] . | Normal [n (%)] . | Fisher's exact (P) . | OR crude (95% CI) . | OR adjusted (95% CI)b . | Nc . |
|---|---|---|---|---|---|---|---|
| Denmark | |||||||
| Use of mild analgesics during pregnancy | |||||||
| No | 7.0 | 15 (35.7) | 200 (44.5) | 0.33 | 1.45 (0.75–2.79) | 1.43 (0.73–2.79) | 491 |
| Yes | 9.8 | 27 (64.3) | 249 (55.5) | ||||
| Use of specific compounds during pregnancy | |||||||
| Paracetamol | |||||||
| No | 7.4 | 19 (45.2) | 238 (53.1) | 0.34 | 1.37 (0.73–2.59) | 1.337 (0.70–2.55) | 490 |
| Yes | 9.9 | 23 (54.8) | 210 (46.9) | ||||
| Acetylsalicylic acid | |||||||
| No | 8.0 | 36 (85.7) | 416 (92.9) | 0.12 | 2.17 (0.85–5.53) | 2.22 (0.86–5.76) | 490 |
| Yes | 15.8 | 6 (14.3) | 32 (7.1) | ||||
| Ibuprofen | |||||||
| No | 8.3 | 39 (92.9) | 430 (96) | 0.411 | 1.84 (0.52–6.51) | 1.82 (0.5–6.61) | 490 |
| Yes | 14.0 | 3 (7.1) | 18 (4) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 7.9 | 38 (90.5) | 440 (98.5) | 0.007 | 7.72 (2.09–28.6) | 7.55 (1.94–29.3) | 488 |
| Yes | 40 | 4 (9.5) | 6 (1.3) | ||||
| Use of mild analgesics during first trimester | |||||||
| No | 8.5 | 26 (74.3) | 280 (80.0) | 0.39 | 1.39 (0.62–3.09) | 1.48 (0.66–3.34) | 385 |
| Yes | 11.4 | 9 (25.7) | 70 (20.0) | ||||
| Use of specific compounds during first trimester | |||||||
| Paracetamol | |||||||
| No | 8.5 | 29 (80.6) | 313 (86.5) | 0.319 | 1.54 (0.64–3.71) | 1.61 (0.66–3.90) | 398 |
| Yes | 12.5 | 7 (19.4) | 49 (13.5) | ||||
| Acetylsalicylic acid | |||||||
| No | 8.0 | 37 (90.2) | 423 (96.8) | 0.059 | 3.27 (1.02–10.4) | 5.60 (1.83–17.1) | 478 |
| Yes | 22.2 | 4 (9.8) | 14 (3.2) | ||||
| Ibuprofen | |||||||
| No | 8.8 | 42 (100) | 434 (97.7) | – | – | – | 486 |
| Yes | 0.0 | 0 (0) | 10 (2.3) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 8.3 | 40 (95.2) | 442 (99.1) | 0.087 | 5.53 (0.89–31.1) | 5.63 (0.98–32.4) | 488 |
| Yes | 33.3 | 2 (4.8) | 4 (0.9) | ||||
| Use of mild analgesics during second trimester | |||||||
| No | 7 | 19 (54.3) | 253 (72.3) | 0.032 | 2.20 (1.09–4.45) | 2.30 (1.12–4.73) | 385 |
| Yes | 14.2 | 16 (45.7) | 97 (27.7) | ||||
| Use of specific compounds during second trimester | |||||||
| Paracetamol | |||||||
| No | 7.6 | 23 (63.9) | 280 (77.3) | 0.099 | 1.93 (0.94–3.98) | 1.97 (0.94–4.12) | 398 |
| Yes | 13.7 | 13 (36.1) | 82 (22.7) | ||||
| Acetylsalicylic acid | |||||||
| No | 7.8 | 36 (87.8) | 425 (97.3) | 0.01 | 4.92 (1.64–14.7) | 3.76 (1.15–12.3) | 478 |
| Yes | 29.4 | 5 (12.2) | 12 (2.7) | ||||
| Ibuprofen | |||||||
| No | 8.2 | 39 (92.9) | 437 (98.4) | 0.047 | 4.8 (1.19–19.3) | 4.59 (1.1–19) | 486 |
| Yes | 30.0 | 3 (7.1) | 7 (1.6) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 7.9 | 38 (90.5) | 443 (99.3) | 0.001 | 15.5 (3.36 – 72) | 16.1 (3.29–78.6) | 488 |
| Yes | 57.4 | 4 (9.5) | 3 (0.70) | ||||
| Finland | |||||||
| Use of mild analgesics during pregnancy | |||||||
| No | 2.6 | 22 (62.9) | 822 (57.6) | 0.61 | 0.80 (0.40–1.60) | 0.74 (0.35–1.57) | 1463 |
| Yes | 2.1 | 13 (37.1) | 606 (42.4) | ||||
| Use of mild analgesics during first trimester | |||||||
| No | 2.6 | 28 (84.8) | 1057 (84.4) | 1.00 | 0.96 (0.37–2.52) | 0.77 (0.26–2.27) | 1286 |
| Yes | 2.5 | 5 (15.2) | 196 (15.6) | ||||
| Use of mild analgesics during second trimester | |||||||
| No | 2.4 | 24 (72.7) | 972 (77.6) | 0.53 | 1.30 (0.60–2.82) | 1.21 (0.53–2.76) | 1286 |
| Yes | 3.1 | 9 (27.3) | 281 (22.4) | ||||
| . | Prevalence (%) . | Cryptorchidisma [n (%)] . | Normal [n (%)] . | Fisher's exact (P) . | OR crude (95% CI) . | OR adjusted (95% CI)b . | Nc . |
|---|---|---|---|---|---|---|---|
| Denmark | |||||||
| Use of mild analgesics during pregnancy | |||||||
| No | 7.0 | 15 (35.7) | 200 (44.5) | 0.33 | 1.45 (0.75–2.79) | 1.43 (0.73–2.79) | 491 |
| Yes | 9.8 | 27 (64.3) | 249 (55.5) | ||||
| Use of specific compounds during pregnancy | |||||||
| Paracetamol | |||||||
| No | 7.4 | 19 (45.2) | 238 (53.1) | 0.34 | 1.37 (0.73–2.59) | 1.337 (0.70–2.55) | 490 |
| Yes | 9.9 | 23 (54.8) | 210 (46.9) | ||||
| Acetylsalicylic acid | |||||||
| No | 8.0 | 36 (85.7) | 416 (92.9) | 0.12 | 2.17 (0.85–5.53) | 2.22 (0.86–5.76) | 490 |
| Yes | 15.8 | 6 (14.3) | 32 (7.1) | ||||
| Ibuprofen | |||||||
| No | 8.3 | 39 (92.9) | 430 (96) | 0.411 | 1.84 (0.52–6.51) | 1.82 (0.5–6.61) | 490 |
| Yes | 14.0 | 3 (7.1) | 18 (4) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 7.9 | 38 (90.5) | 440 (98.5) | 0.007 | 7.72 (2.09–28.6) | 7.55 (1.94–29.3) | 488 |
| Yes | 40 | 4 (9.5) | 6 (1.3) | ||||
| Use of mild analgesics during first trimester | |||||||
| No | 8.5 | 26 (74.3) | 280 (80.0) | 0.39 | 1.39 (0.62–3.09) | 1.48 (0.66–3.34) | 385 |
| Yes | 11.4 | 9 (25.7) | 70 (20.0) | ||||
| Use of specific compounds during first trimester | |||||||
| Paracetamol | |||||||
| No | 8.5 | 29 (80.6) | 313 (86.5) | 0.319 | 1.54 (0.64–3.71) | 1.61 (0.66–3.90) | 398 |
| Yes | 12.5 | 7 (19.4) | 49 (13.5) | ||||
| Acetylsalicylic acid | |||||||
| No | 8.0 | 37 (90.2) | 423 (96.8) | 0.059 | 3.27 (1.02–10.4) | 5.60 (1.83–17.1) | 478 |
| Yes | 22.2 | 4 (9.8) | 14 (3.2) | ||||
| Ibuprofen | |||||||
| No | 8.8 | 42 (100) | 434 (97.7) | – | – | – | 486 |
| Yes | 0.0 | 0 (0) | 10 (2.3) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 8.3 | 40 (95.2) | 442 (99.1) | 0.087 | 5.53 (0.89–31.1) | 5.63 (0.98–32.4) | 488 |
| Yes | 33.3 | 2 (4.8) | 4 (0.9) | ||||
| Use of mild analgesics during second trimester | |||||||
| No | 7 | 19 (54.3) | 253 (72.3) | 0.032 | 2.20 (1.09–4.45) | 2.30 (1.12–4.73) | 385 |
| Yes | 14.2 | 16 (45.7) | 97 (27.7) | ||||
| Use of specific compounds during second trimester | |||||||
| Paracetamol | |||||||
| No | 7.6 | 23 (63.9) | 280 (77.3) | 0.099 | 1.93 (0.94–3.98) | 1.97 (0.94–4.12) | 398 |
| Yes | 13.7 | 13 (36.1) | 82 (22.7) | ||||
| Acetylsalicylic acid | |||||||
| No | 7.8 | 36 (87.8) | 425 (97.3) | 0.01 | 4.92 (1.64–14.7) | 3.76 (1.15–12.3) | 478 |
| Yes | 29.4 | 5 (12.2) | 12 (2.7) | ||||
| Ibuprofen | |||||||
| No | 8.2 | 39 (92.9) | 437 (98.4) | 0.047 | 4.8 (1.19–19.3) | 4.59 (1.1–19) | 486 |
| Yes | 30.0 | 3 (7.1) | 7 (1.6) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 7.9 | 38 (90.5) | 443 (99.3) | 0.001 | 15.5 (3.36 – 72) | 16.1 (3.29–78.6) | 488 |
| Yes | 57.4 | 4 (9.5) | 3 (0.70) | ||||
| Finland | |||||||
| Use of mild analgesics during pregnancy | |||||||
| No | 2.6 | 22 (62.9) | 822 (57.6) | 0.61 | 0.80 (0.40–1.60) | 0.74 (0.35–1.57) | 1463 |
| Yes | 2.1 | 13 (37.1) | 606 (42.4) | ||||
| Use of mild analgesics during first trimester | |||||||
| No | 2.6 | 28 (84.8) | 1057 (84.4) | 1.00 | 0.96 (0.37–2.52) | 0.77 (0.26–2.27) | 1286 |
| Yes | 2.5 | 5 (15.2) | 196 (15.6) | ||||
| Use of mild analgesics during second trimester | |||||||
| No | 2.4 | 24 (72.7) | 972 (77.6) | 0.53 | 1.30 (0.60–2.82) | 1.21 (0.53–2.76) | 1286 |
| Yes | 3.1 | 9 (27.3) | 281 (22.4) | ||||
aTestis defined as cryptorchid if it was high scrotal, supra-scrotal, inguinal and non-palpable.
bAdjusted for gestational age, reported disease and use of other medicine during pregnancy.
cThe numbers differ since not all women provided information about the trimester of use.
Maternal use of mild analgesics during pregnancy in relation to congenital cryptorchidism.
| . | Prevalence (%) . | Cryptorchidisma [n (%)] . | Normal [n (%)] . | Fisher's exact (P) . | OR crude (95% CI) . | OR adjusted (95% CI)b . | Nc . |
|---|---|---|---|---|---|---|---|
| Denmark | |||||||
| Use of mild analgesics during pregnancy | |||||||
| No | 7.0 | 15 (35.7) | 200 (44.5) | 0.33 | 1.45 (0.75–2.79) | 1.43 (0.73–2.79) | 491 |
| Yes | 9.8 | 27 (64.3) | 249 (55.5) | ||||
| Use of specific compounds during pregnancy | |||||||
| Paracetamol | |||||||
| No | 7.4 | 19 (45.2) | 238 (53.1) | 0.34 | 1.37 (0.73–2.59) | 1.337 (0.70–2.55) | 490 |
| Yes | 9.9 | 23 (54.8) | 210 (46.9) | ||||
| Acetylsalicylic acid | |||||||
| No | 8.0 | 36 (85.7) | 416 (92.9) | 0.12 | 2.17 (0.85–5.53) | 2.22 (0.86–5.76) | 490 |
| Yes | 15.8 | 6 (14.3) | 32 (7.1) | ||||
| Ibuprofen | |||||||
| No | 8.3 | 39 (92.9) | 430 (96) | 0.411 | 1.84 (0.52–6.51) | 1.82 (0.5–6.61) | 490 |
| Yes | 14.0 | 3 (7.1) | 18 (4) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 7.9 | 38 (90.5) | 440 (98.5) | 0.007 | 7.72 (2.09–28.6) | 7.55 (1.94–29.3) | 488 |
| Yes | 40 | 4 (9.5) | 6 (1.3) | ||||
| Use of mild analgesics during first trimester | |||||||
| No | 8.5 | 26 (74.3) | 280 (80.0) | 0.39 | 1.39 (0.62–3.09) | 1.48 (0.66–3.34) | 385 |
| Yes | 11.4 | 9 (25.7) | 70 (20.0) | ||||
| Use of specific compounds during first trimester | |||||||
| Paracetamol | |||||||
| No | 8.5 | 29 (80.6) | 313 (86.5) | 0.319 | 1.54 (0.64–3.71) | 1.61 (0.66–3.90) | 398 |
| Yes | 12.5 | 7 (19.4) | 49 (13.5) | ||||
| Acetylsalicylic acid | |||||||
| No | 8.0 | 37 (90.2) | 423 (96.8) | 0.059 | 3.27 (1.02–10.4) | 5.60 (1.83–17.1) | 478 |
| Yes | 22.2 | 4 (9.8) | 14 (3.2) | ||||
| Ibuprofen | |||||||
| No | 8.8 | 42 (100) | 434 (97.7) | – | – | – | 486 |
| Yes | 0.0 | 0 (0) | 10 (2.3) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 8.3 | 40 (95.2) | 442 (99.1) | 0.087 | 5.53 (0.89–31.1) | 5.63 (0.98–32.4) | 488 |
| Yes | 33.3 | 2 (4.8) | 4 (0.9) | ||||
| Use of mild analgesics during second trimester | |||||||
| No | 7 | 19 (54.3) | 253 (72.3) | 0.032 | 2.20 (1.09–4.45) | 2.30 (1.12–4.73) | 385 |
| Yes | 14.2 | 16 (45.7) | 97 (27.7) | ||||
| Use of specific compounds during second trimester | |||||||
| Paracetamol | |||||||
| No | 7.6 | 23 (63.9) | 280 (77.3) | 0.099 | 1.93 (0.94–3.98) | 1.97 (0.94–4.12) | 398 |
| Yes | 13.7 | 13 (36.1) | 82 (22.7) | ||||
| Acetylsalicylic acid | |||||||
| No | 7.8 | 36 (87.8) | 425 (97.3) | 0.01 | 4.92 (1.64–14.7) | 3.76 (1.15–12.3) | 478 |
| Yes | 29.4 | 5 (12.2) | 12 (2.7) | ||||
| Ibuprofen | |||||||
| No | 8.2 | 39 (92.9) | 437 (98.4) | 0.047 | 4.8 (1.19–19.3) | 4.59 (1.1–19) | 486 |
| Yes | 30.0 | 3 (7.1) | 7 (1.6) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 7.9 | 38 (90.5) | 443 (99.3) | 0.001 | 15.5 (3.36 – 72) | 16.1 (3.29–78.6) | 488 |
| Yes | 57.4 | 4 (9.5) | 3 (0.70) | ||||
| Finland | |||||||
| Use of mild analgesics during pregnancy | |||||||
| No | 2.6 | 22 (62.9) | 822 (57.6) | 0.61 | 0.80 (0.40–1.60) | 0.74 (0.35–1.57) | 1463 |
| Yes | 2.1 | 13 (37.1) | 606 (42.4) | ||||
| Use of mild analgesics during first trimester | |||||||
| No | 2.6 | 28 (84.8) | 1057 (84.4) | 1.00 | 0.96 (0.37–2.52) | 0.77 (0.26–2.27) | 1286 |
| Yes | 2.5 | 5 (15.2) | 196 (15.6) | ||||
| Use of mild analgesics during second trimester | |||||||
| No | 2.4 | 24 (72.7) | 972 (77.6) | 0.53 | 1.30 (0.60–2.82) | 1.21 (0.53–2.76) | 1286 |
| Yes | 3.1 | 9 (27.3) | 281 (22.4) | ||||
| . | Prevalence (%) . | Cryptorchidisma [n (%)] . | Normal [n (%)] . | Fisher's exact (P) . | OR crude (95% CI) . | OR adjusted (95% CI)b . | Nc . |
|---|---|---|---|---|---|---|---|
| Denmark | |||||||
| Use of mild analgesics during pregnancy | |||||||
| No | 7.0 | 15 (35.7) | 200 (44.5) | 0.33 | 1.45 (0.75–2.79) | 1.43 (0.73–2.79) | 491 |
| Yes | 9.8 | 27 (64.3) | 249 (55.5) | ||||
| Use of specific compounds during pregnancy | |||||||
| Paracetamol | |||||||
| No | 7.4 | 19 (45.2) | 238 (53.1) | 0.34 | 1.37 (0.73–2.59) | 1.337 (0.70–2.55) | 490 |
| Yes | 9.9 | 23 (54.8) | 210 (46.9) | ||||
| Acetylsalicylic acid | |||||||
| No | 8.0 | 36 (85.7) | 416 (92.9) | 0.12 | 2.17 (0.85–5.53) | 2.22 (0.86–5.76) | 490 |
| Yes | 15.8 | 6 (14.3) | 32 (7.1) | ||||
| Ibuprofen | |||||||
| No | 8.3 | 39 (92.9) | 430 (96) | 0.411 | 1.84 (0.52–6.51) | 1.82 (0.5–6.61) | 490 |
| Yes | 14.0 | 3 (7.1) | 18 (4) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 7.9 | 38 (90.5) | 440 (98.5) | 0.007 | 7.72 (2.09–28.6) | 7.55 (1.94–29.3) | 488 |
| Yes | 40 | 4 (9.5) | 6 (1.3) | ||||
| Use of mild analgesics during first trimester | |||||||
| No | 8.5 | 26 (74.3) | 280 (80.0) | 0.39 | 1.39 (0.62–3.09) | 1.48 (0.66–3.34) | 385 |
| Yes | 11.4 | 9 (25.7) | 70 (20.0) | ||||
| Use of specific compounds during first trimester | |||||||
| Paracetamol | |||||||
| No | 8.5 | 29 (80.6) | 313 (86.5) | 0.319 | 1.54 (0.64–3.71) | 1.61 (0.66–3.90) | 398 |
| Yes | 12.5 | 7 (19.4) | 49 (13.5) | ||||
| Acetylsalicylic acid | |||||||
| No | 8.0 | 37 (90.2) | 423 (96.8) | 0.059 | 3.27 (1.02–10.4) | 5.60 (1.83–17.1) | 478 |
| Yes | 22.2 | 4 (9.8) | 14 (3.2) | ||||
| Ibuprofen | |||||||
| No | 8.8 | 42 (100) | 434 (97.7) | – | – | – | 486 |
| Yes | 0.0 | 0 (0) | 10 (2.3) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 8.3 | 40 (95.2) | 442 (99.1) | 0.087 | 5.53 (0.89–31.1) | 5.63 (0.98–32.4) | 488 |
| Yes | 33.3 | 2 (4.8) | 4 (0.9) | ||||
| Use of mild analgesics during second trimester | |||||||
| No | 7 | 19 (54.3) | 253 (72.3) | 0.032 | 2.20 (1.09–4.45) | 2.30 (1.12–4.73) | 385 |
| Yes | 14.2 | 16 (45.7) | 97 (27.7) | ||||
| Use of specific compounds during second trimester | |||||||
| Paracetamol | |||||||
| No | 7.6 | 23 (63.9) | 280 (77.3) | 0.099 | 1.93 (0.94–3.98) | 1.97 (0.94–4.12) | 398 |
| Yes | 13.7 | 13 (36.1) | 82 (22.7) | ||||
| Acetylsalicylic acid | |||||||
| No | 7.8 | 36 (87.8) | 425 (97.3) | 0.01 | 4.92 (1.64–14.7) | 3.76 (1.15–12.3) | 478 |
| Yes | 29.4 | 5 (12.2) | 12 (2.7) | ||||
| Ibuprofen | |||||||
| No | 8.2 | 39 (92.9) | 437 (98.4) | 0.047 | 4.8 (1.19–19.3) | 4.59 (1.1–19) | 486 |
| Yes | 30.0 | 3 (7.1) | 7 (1.6) | ||||
| Simultaneous use of >1 compound | |||||||
| No | 7.9 | 38 (90.5) | 443 (99.3) | 0.001 | 15.5 (3.36 – 72) | 16.1 (3.29–78.6) | 488 |
| Yes | 57.4 | 4 (9.5) | 3 (0.70) | ||||
| Finland | |||||||
| Use of mild analgesics during pregnancy | |||||||
| No | 2.6 | 22 (62.9) | 822 (57.6) | 0.61 | 0.80 (0.40–1.60) | 0.74 (0.35–1.57) | 1463 |
| Yes | 2.1 | 13 (37.1) | 606 (42.4) | ||||
| Use of mild analgesics during first trimester | |||||||
| No | 2.6 | 28 (84.8) | 1057 (84.4) | 1.00 | 0.96 (0.37–2.52) | 0.77 (0.26–2.27) | 1286 |
| Yes | 2.5 | 5 (15.2) | 196 (15.6) | ||||
| Use of mild analgesics during second trimester | |||||||
| No | 2.4 | 24 (72.7) | 972 (77.6) | 0.53 | 1.30 (0.60–2.82) | 1.21 (0.53–2.76) | 1286 |
| Yes | 3.1 | 9 (27.3) | 281 (22.4) | ||||
aTestis defined as cryptorchid if it was high scrotal, supra-scrotal, inguinal and non-palpable.
bAdjusted for gestational age, reported disease and use of other medicine during pregnancy.
cThe numbers differ since not all women provided information about the trimester of use.
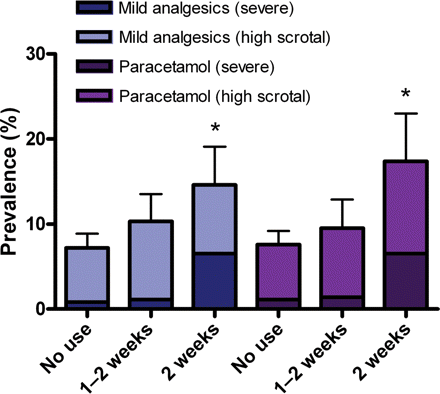
Use during the second trimester in particular increased the risk of congenital cryptorchidism [adjusted OR 2.3 (1.12–4.73), P = 0.032]. The risk remained significant for the individual compounds ibuprofen and acetylsalicylic acid and showed the same trend for paracetamol. The risk was further increased among mothers who reported the simultaneous use of more than one analgesic [adjusted OR 16.1 (3.29–78.6), P = 0.001]. Separating the cases with cryptorchidism into high scrotal and more severe forms showed an increased risk in both categories after the maternal use of mild analgesics (Fig. 2).
Mean prevalence of congenital cryptorchidism relative to weeks of maternal use of any mild analgesics and paracetamol during the first and the second trimester. *P < 0.05. P-values are between mothers using no compounds and analgesic use for >2 weeks and are adjusted for disease, use of other medicine and gestational age using logistic regression. Error bars are ±1.96 SE.
To test whether the risk of cryptorchidism was associated with the extent of analgesic use, we categorized the mothers into three groups, consisting of (i) no use, (ii) use for 1–2 weeks and (iii) use for more than 2 weeks during first and second trimester. Mothers who reported intake of mild analgesics for more than 2 weeks had a significantly increased risk of giving birth to boys born with cryptorchidism [adjusted OR 2.47 (1.02–5.96), P = 0.045], with similar individual results for paracetamol and acetylsalicylic acid (Fig. 2 and Table II). The highest risk was observed among the small number of mothers who used more than one compound simultaneously for more than 2 weeks [adjusted OR 21.7 (1.83–258), P = 0.015].
Extent of maternal use of mild analgesics in first and second trimester of pregnancy in relation to congenital cryptorchidism.
| Compound . | Weeks of usage . | Na . | Cryptorchidism (%)b . | OR crude (95% CI) . | OR adjusted (95% CI)c . |
|---|---|---|---|---|---|
| Denmark | |||||
| Mild analgesics | 0 | 236 | 7.2 | 1 | 1 |
| 1–2 | 87 | 10.3 | 1.49 (0.64–3.47) | 1.5 (0.63–3.55) | |
| >2 | 62 | 14.5 | 2.19 (0.92–5.18) | 2.47 (1.02–5.96) | |
| Paracetamol | 0 | 278 | 7.6 | 1 | 1 |
| 1–2 | 74 | 9.5 | 1.28 (0.52–3.13) | 1.26 (0.5–3.14) | |
| >2 | 46 | 17.4 | 2.58 (1.07–6.23) | 2.78 (1.13–6.84) | |
| Acetylsalicylic acid | 0 | 454 | 7.9 | 1 | 1 |
| 1–2 | 11 | 18.2 | 2.58 (0.54–12.4) | 2.75 (0.56–13.5) | |
| >2 | 13 | 23.1 | 3.35 (0.92–13.2) | 4.07 (1.05–15.8) | |
| Ibuprofen | 0 | 470 | 8.3 | 1 | 1 |
| 1–2 | 11 | 27.3 | 4.14 (1.06–16.3) | 3.85 (0.93–15.9) | |
| >2 | 5 | 0 | – | – | |
| Use of >1 compound | 0 | 478 | 7.9 | 1 | 1 |
| 1–2 | 7 | 28.6 | 4.63 (0.87–24.7) | 4.63 (0.83–52.8) | |
| >2 | 3 | 66.7 | 23.16 (2.05–261) | 21.69 (1.83–258) | |
| Finland | |||||
| Mild analgesics | 0 | 905 | 2.5 | 1 | 1 |
| 1–2 | 231 | 3.0 | 1.20 (0.51–2.83) | 1.21 (0.50–2.90) | |
| >2 | 150 | 2.0 | 0.78 (0.23–2.64) | 0.56 (0.13–2.45) | |
| Paracetamol | 0 | 945 | 2.5 | 1 | 1 |
| 1–2 | 214 | 2.8 | 1.11 (0.45–2.74) | 1.01 (0.44–2.79) | |
| >2 | 131 | 2.3 | 0.90 (0.27–3.03) | 0.64 (0.15–2.79) | |
| Compound . | Weeks of usage . | Na . | Cryptorchidism (%)b . | OR crude (95% CI) . | OR adjusted (95% CI)c . |
|---|---|---|---|---|---|
| Denmark | |||||
| Mild analgesics | 0 | 236 | 7.2 | 1 | 1 |
| 1–2 | 87 | 10.3 | 1.49 (0.64–3.47) | 1.5 (0.63–3.55) | |
| >2 | 62 | 14.5 | 2.19 (0.92–5.18) | 2.47 (1.02–5.96) | |
| Paracetamol | 0 | 278 | 7.6 | 1 | 1 |
| 1–2 | 74 | 9.5 | 1.28 (0.52–3.13) | 1.26 (0.5–3.14) | |
| >2 | 46 | 17.4 | 2.58 (1.07–6.23) | 2.78 (1.13–6.84) | |
| Acetylsalicylic acid | 0 | 454 | 7.9 | 1 | 1 |
| 1–2 | 11 | 18.2 | 2.58 (0.54–12.4) | 2.75 (0.56–13.5) | |
| >2 | 13 | 23.1 | 3.35 (0.92–13.2) | 4.07 (1.05–15.8) | |
| Ibuprofen | 0 | 470 | 8.3 | 1 | 1 |
| 1–2 | 11 | 27.3 | 4.14 (1.06–16.3) | 3.85 (0.93–15.9) | |
| >2 | 5 | 0 | – | – | |
| Use of >1 compound | 0 | 478 | 7.9 | 1 | 1 |
| 1–2 | 7 | 28.6 | 4.63 (0.87–24.7) | 4.63 (0.83–52.8) | |
| >2 | 3 | 66.7 | 23.16 (2.05–261) | 21.69 (1.83–258) | |
| Finland | |||||
| Mild analgesics | 0 | 905 | 2.5 | 1 | 1 |
| 1–2 | 231 | 3.0 | 1.20 (0.51–2.83) | 1.21 (0.50–2.90) | |
| >2 | 150 | 2.0 | 0.78 (0.23–2.64) | 0.56 (0.13–2.45) | |
| Paracetamol | 0 | 945 | 2.5 | 1 | 1 |
| 1–2 | 214 | 2.8 | 1.11 (0.45–2.74) | 1.01 (0.44–2.79) | |
| >2 | 131 | 2.3 | 0.90 (0.27–3.03) | 0.64 (0.15–2.79) | |
aThe numbers differ between the different mild analgesics since not all women provided information about duration of use.
bTestis defined as cryptorchid if it was high scrotal, supra-scrotal, inguinal and non-palpable.
cAdjusted for gestational age, reported disease and use of other medicine during pregnancy.
Extent of maternal use of mild analgesics in first and second trimester of pregnancy in relation to congenital cryptorchidism.
| Compound . | Weeks of usage . | Na . | Cryptorchidism (%)b . | OR crude (95% CI) . | OR adjusted (95% CI)c . |
|---|---|---|---|---|---|
| Denmark | |||||
| Mild analgesics | 0 | 236 | 7.2 | 1 | 1 |
| 1–2 | 87 | 10.3 | 1.49 (0.64–3.47) | 1.5 (0.63–3.55) | |
| >2 | 62 | 14.5 | 2.19 (0.92–5.18) | 2.47 (1.02–5.96) | |
| Paracetamol | 0 | 278 | 7.6 | 1 | 1 |
| 1–2 | 74 | 9.5 | 1.28 (0.52–3.13) | 1.26 (0.5–3.14) | |
| >2 | 46 | 17.4 | 2.58 (1.07–6.23) | 2.78 (1.13–6.84) | |
| Acetylsalicylic acid | 0 | 454 | 7.9 | 1 | 1 |
| 1–2 | 11 | 18.2 | 2.58 (0.54–12.4) | 2.75 (0.56–13.5) | |
| >2 | 13 | 23.1 | 3.35 (0.92–13.2) | 4.07 (1.05–15.8) | |
| Ibuprofen | 0 | 470 | 8.3 | 1 | 1 |
| 1–2 | 11 | 27.3 | 4.14 (1.06–16.3) | 3.85 (0.93–15.9) | |
| >2 | 5 | 0 | – | – | |
| Use of >1 compound | 0 | 478 | 7.9 | 1 | 1 |
| 1–2 | 7 | 28.6 | 4.63 (0.87–24.7) | 4.63 (0.83–52.8) | |
| >2 | 3 | 66.7 | 23.16 (2.05–261) | 21.69 (1.83–258) | |
| Finland | |||||
| Mild analgesics | 0 | 905 | 2.5 | 1 | 1 |
| 1–2 | 231 | 3.0 | 1.20 (0.51–2.83) | 1.21 (0.50–2.90) | |
| >2 | 150 | 2.0 | 0.78 (0.23–2.64) | 0.56 (0.13–2.45) | |
| Paracetamol | 0 | 945 | 2.5 | 1 | 1 |
| 1–2 | 214 | 2.8 | 1.11 (0.45–2.74) | 1.01 (0.44–2.79) | |
| >2 | 131 | 2.3 | 0.90 (0.27–3.03) | 0.64 (0.15–2.79) | |
| Compound . | Weeks of usage . | Na . | Cryptorchidism (%)b . | OR crude (95% CI) . | OR adjusted (95% CI)c . |
|---|---|---|---|---|---|
| Denmark | |||||
| Mild analgesics | 0 | 236 | 7.2 | 1 | 1 |
| 1–2 | 87 | 10.3 | 1.49 (0.64–3.47) | 1.5 (0.63–3.55) | |
| >2 | 62 | 14.5 | 2.19 (0.92–5.18) | 2.47 (1.02–5.96) | |
| Paracetamol | 0 | 278 | 7.6 | 1 | 1 |
| 1–2 | 74 | 9.5 | 1.28 (0.52–3.13) | 1.26 (0.5–3.14) | |
| >2 | 46 | 17.4 | 2.58 (1.07–6.23) | 2.78 (1.13–6.84) | |
| Acetylsalicylic acid | 0 | 454 | 7.9 | 1 | 1 |
| 1–2 | 11 | 18.2 | 2.58 (0.54–12.4) | 2.75 (0.56–13.5) | |
| >2 | 13 | 23.1 | 3.35 (0.92–13.2) | 4.07 (1.05–15.8) | |
| Ibuprofen | 0 | 470 | 8.3 | 1 | 1 |
| 1–2 | 11 | 27.3 | 4.14 (1.06–16.3) | 3.85 (0.93–15.9) | |
| >2 | 5 | 0 | – | – | |
| Use of >1 compound | 0 | 478 | 7.9 | 1 | 1 |
| 1–2 | 7 | 28.6 | 4.63 (0.87–24.7) | 4.63 (0.83–52.8) | |
| >2 | 3 | 66.7 | 23.16 (2.05–261) | 21.69 (1.83–258) | |
| Finland | |||||
| Mild analgesics | 0 | 905 | 2.5 | 1 | 1 |
| 1–2 | 231 | 3.0 | 1.20 (0.51–2.83) | 1.21 (0.50–2.90) | |
| >2 | 150 | 2.0 | 0.78 (0.23–2.64) | 0.56 (0.13–2.45) | |
| Paracetamol | 0 | 945 | 2.5 | 1 | 1 |
| 1–2 | 214 | 2.8 | 1.11 (0.45–2.74) | 1.01 (0.44–2.79) | |
| >2 | 131 | 2.3 | 0.90 (0.27–3.03) | 0.64 (0.15–2.79) | |
aThe numbers differ between the different mild analgesics since not all women provided information about duration of use.
bTestis defined as cryptorchid if it was high scrotal, supra-scrotal, inguinal and non-palpable.
cAdjusted for gestational age, reported disease and use of other medicine during pregnancy.
Despite underreporting, results from the Danish written questionnaire showed a trend in the same direction (Supplementary data, Tables SI and SII). However, based on the questionnaire data, the use of analgesic medicine was in general not associated with congenital cryptorchidism in the Finnish cohort, except for a trend in the second trimester (Tables I and II). We did not find a significant association between the use of mild analgesics and hypospadia in the two birth cohorts.
Intrauterine rat model
To verify the association seen in the prospective birth cohort between mild analgesics and the altered testis descent, we extended the investigation with the use of intrauterine exposure experiments in the rat with AGD as end-point as this is a more sensitive marker for reduced intrauterine androgen levels than cryptorchidism (Welsh et al., 2008). No clinical signs of general toxicity were observed during the daily observations. At GD21, the pregnant dams were sacrificed and examined together with the fetuses. No signs of liver toxicity were detected. Maternal body weight gain, litter sizes, number of live fetuses, resorptions and implantation as well as the sex ratio in the litters were not affected in the dosed groups when compared with controls (Supplementary data, Table SIII).
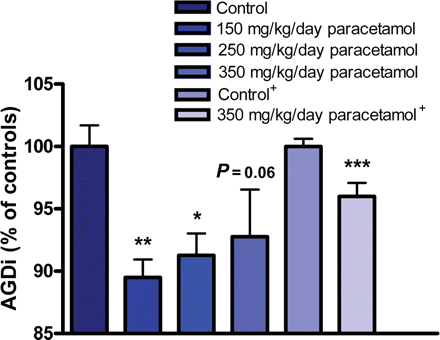
Intrauterine exposure to paracetamol resulted in reduced AGDi in male fetuses for all three dose groups (Fig. 3 and Supplementary data, Table SIV). To ascertain these results, we performed an additional and independent study with a larger group of animals, using only the top dose of 350 mg/kg/day paracetamol. Consistent with the previous data, this experiment showed a significant reduction in AGDi in the male fetuses. When the data from the two paracetamol studies were combined, including study number as a factor in the statistical analysis, the significance of reductions in AGD and AGDi for all three dose groups was further increased (data not shown). Furthermore, when testosterone production by the fetal testes was assessed, we found a non-significant reduction for the highest doses in both experiments compared with the unexposed control groups (Supplementary data, Table SV).
Intrauterine exposure from GD13 to 21 to paracetamol in rats led to a reduction in the testosterone-dependent AGD of male offspring. Results from initial experiment performed with four to five litters per dose group and the verifying experiment (+) performed with six litters per dose group. ***P < 0.001, **P < 0.01, *P < 0.05. P-values are from ANOVA followed by Dunnett's post hoc test with litter as the statistical unit and included as a random factor in a mixed model. There is no significant difference between treated groups. Data represent the mean ± SEM.
We also performed an intrauterine exposure study with acetylsalicylic acid, which resulted in shorter AGD in all male fetuses compared with controls. However, as expected from previous experiments (Gupta et al., 2003), acetylsalicylic acid also resulted in intrauterine fetal growth retardation to such a degree that the difference in AGD was undetectable after adjusting for body weight (Supplementary data, Table SIV). Examination of testosterone production by the testes exposed in utero to acetylsalicylic acid showed a significant (P < 0.05) and not significant (P = 0.065 and 0.077) reduction, compared with unexposed control testes (Supplementary data, Table SV).
Ex vivo rat model
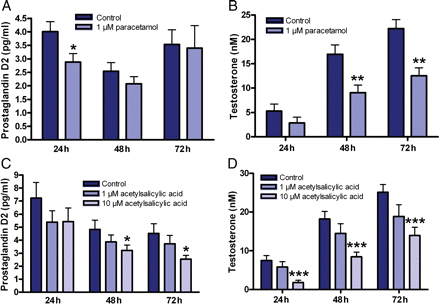
Since intrauterine androgens are required for normal abdominal translocation of the testes (Boisen et al., 2004; Tomiyama et al., 2005; Swan et al., 2005; Scott et al., 2009) and decreased AGD is a indicator for reduced intrauterine androgen levels (Welsh et al., 2008), we next used organotypic culture of fetal rat testes to examine effect of mild analgesics on fetal testosterone production. The ex vivo system supports normal differentiation of fetal rat gonads as reflected by the progressive increase in testosterone production and has been validated for toxicological studies (Habert et al., 1991; Lassurguere et al., 2003; Chauvigne et al., 2009). The increase in testosterone production indicates that the number of Leydig cells continued to increase and/or mature throughout the 3 days of culture during the masculinization window (Chauvigne et al., 2009). The mild analgesics reduced PGD2 production (Fig. 4A and 4C) and in the presence of 1 µM paracetamol secretion of testosterone were consistently reduced by ∼50% throughout the assay period (Fig. 4B). A similar dose-dependent reduction in testosterone was seen with 10 µM acetylsalicylic acid at all time points with statistical significance at a dose of 10 µM (Fig. 4D).
Mild analgesic compounds paracetamol and acetylsalicylic acid reduced PGD2 and testosterone production in ex vivo cultured GD14.5 rat testes. (A) Paracetamol (n = 10) and (C) acetylsalicylic acid (n = 8) reduced PGD2 secretion during ex vivo culture. (B) Paracetamol (n = 10) and (D) acetylsalicylic acid (n = 13) reduced testosterone secretion from the fetal rat testes during 3 days of ex vivo culture. ***P < 0.001, **P < 0.01, *P < 0.05. P-values are from a two-sided unpaired Student's t-test. Data represent the mean ± SEM.
Discussion
In this comprehensive study, we found a direct association between both the timing and extent of mild analgesic consumption during pregnancy and the risk of giving birth to a son with congenital cryptorchidism, the best described risk factor for poor semen quality and testicular germ cell cancer (Boisen et al., 2004). Using two different rat models, we further support these data and show that fetal exposure to mild analgesics exerts an anti-androgenic action in the male.
The cohort study was performed by trained paediatricians and incorporated mild cryptorchidism (high scrotal testis) as part of the continuum of maldescent, which is reflected in subtle primary testicular dysfunction (Suomi et al., 2006), setting this study significantly apart from former investigations that focused exclusively on the most severe types of cryptorchidism reported in Hospital registries (Norgard et al., 2005; Ofori et al., 2006; Rebordosa et al., 2008a). The results indicate an increase in prevalence after the maternal use of mild analgesic in both the mild and more severe forms of congenital cryptorchidism. Importantly, data from mothers, who completed both a self-administrated questionnaire and participated in a computer-assisted telephone interview, revealed a pit fall in the questionnaire data since many mothers had not considered mild analgesics as ‘true’ medication and hence underreported their use. The majority of the mothers used the analgesics due to simple pain such as headache and muscle ache, consistent with the fact that adjusting for underlying diseases did not change the association between the use of mild analgesics and cryptorchidism. This indicates that our observations do not result from specific diseases, which encouraged the women to take analgesics.
The association with cryptorchidism was not found in the Finnish cohort, except for a trend in the second trimester. However, the birth prevalence of cryptorchidism in Finland (2.4%) was much lower than that in Denmark (9.0%; Boisen et al., 2004) and our study may be statistically underpowered to find an association. In accordance, the association we find in the Danish cohort was recently supported by an independent Danish study of 47 400 boys with an incidence of cryptorchidism (2%) similar to that observed in the Finnish cohort (Jensen et al., 2010). In that study, they report a significant association between the use of paracetamol in weeks 8–14 and cryptorchidism (Jensen et al., 2010). We did not find an association with hypospadia in any of the birth cohorts; however, the incidence of hypospadia was very low at birth (1% in the Danish and 0.3% in the Finnish cohort) and it is not clear how large the association is between cryptorchidism and hypospadia (Akre and Richiardi, 2009; Jørgensen et al., 2010).
It is widely accepted that androgens are required for normal abdominal translocation of the testes (Boisen et al., 2004; Swan et al., 2005; Tomiyama et al., 2005; Scott et al., 2009). Therefore, a particular strength of this study is the use of two complementary rat models to support the contention that the association between analgesic use and cryptorchidism seen in our cohort study may result from a reduction in androgen production. Intrauterine exposure to paracetamol, with a lowest dose of 150 mg/kg/day, resulted in a decrease in fetal testosterone-dependent AGDi comparable with effects seen after exposure to known anti-androgenic compounds such as phthalates (Borch et al., 2006), which have PG inhibitory properties similar to those of mild analgesics (D. M. Kristensen et al., submitted for publication). Since this is only three times the dose recommended for humans of 50 mg/kg/day, further work is needed to determined the no adverse effect level. Effects after exposure to acetylsalicylic acid in the rats were more severe than after paracetamol and resulted in fetal growth retardation, which is in line with previous reports (Gupta et al., 2003), and reduction in testosterone production as also described in mouse studies (Gupta and Goldman, 1986). Examination of mild analgesics’ effects directly on the initiation of fetal testosterone production in GD14.5 rat testes further supported the notion of a direct anti-androgenic effect. Surprisingly, paracetamol was the most potent inhibitor of testosterone production and had significant inhibitory effect already at 1 µM, which is well below the therapeutically plasma concentration of 65–130 µM. Hence, there was no direct correlation in this assay between the potency of the different mild analgesics on PG synthesis and the inhibition of testosterone. This is in contrast to the human data, where ibuprofen, as the most potent PG synthesis inhibitor, was associated with the highest risk of congenital cryptorchidism. Acetylsalicylic acid has previously been shown to partially block the increase in testosterone production after hCG stimulation of adult men (Conte et al., 1999). A similar scenario is plausible during fetal development, which could account for the anti-androgenic effect. Experiments in rats have shown that decrease in androgen action during a specific masculinization programming window, corresponding approximately to gestational week 8–14 in humans, results in an increase in male reproductive disorders including cryptorchidism (Welsh et al., 2008). This corresponds to the end of the first and the start of the second trimester, which is in accordance with the birth cohort study and supported by the study by Jensen et al. (2010). However, since PGD2 plays a role in early male sexual differentiation (Adams and McLaren, 2002; Wilhelm et al., 2007) and the results show an inhibition of this paracrine factor in the fetal rat testes, it is also possible that there are an additional effect on testes development.
During the late 1950s, a Danish investigation similar to this study in terms of design included 2700 boys born at Rigshospitalet, Copenhagen (Buemann et al., 1961). Comparing the prevalence of congenital cryptorchidism with the data from the present cohort indicate a marked increase in the incidence of this disorder (1.8% in 1959–1961 versus 8.5% in 1997–2001, P < 0.001; Boisen et al., 2004). The magnitude of this difference is too large to be accounted for by random fluctuations and differences in ascertainment. Moreover, this finding is in accordance with the reported decline in reproductive health in the adult male population over the past five decades (Swan et al., 1997; Richiardi et al., 2004). The epidemiological and clinical associations between reproductive health problems such as subfertility, testicular germ cell cancer and cryptorchidism suggest the existence of etiologic and pathogenic links, as suggested in the TDS hypothesis (Skakkebaek et al., 2001). Observations made in wildlife after environmental accidents have yielded substantial evidence of adverse developmental effects caused by endocrine disrupters and studies on phthalates in humans have shown that there is an association between maternal exposure and reduction in AGD and cryptorchidism among newborn boys (Swan et al., 2005). Recent studies also indicate that anti-androgens, which in low concentrations exert no effects, when combined induce reproductive disorders (Hass et al., 2007). Thus, since pregnant women in the Western world are constantly and inevitably exposed to low concentrations of a large number of different anti-androgens (Diamanti-Kandarakis et al., 2009; Scott et al., 2009), of which many are PG synthesis inhibitors (D. M. Kristensen et al., submitted for publication), consumption of mild analgesics such as paracetamol could in combination with exposure to environmental PG inhibitory and anti-androgenic compounds be a contributing factor to the increased incidence of cryptorchidism and later life reproductive problems.
Collectively, the results points to a scenario where the use of mild analgesic medicine has a possible effect on fetal development with implications for later reproductive health. Therefore, more investigations are urgently needed and we will for our part continue to follow the boys in our cohorts, who currently are entering puberty.
Author's contributions
D.M.K. and H.L. initiated the project. D.M.K., H.L., N.E.S., S.B., U.H., K.M.M. and B.J. conceived and designed the experiments. B.J., L.L. and C.D.L. performed the fetal rat testes experiments, and D.M.K. analysed samples and data. U.H., P.R.J., J.B. and C.N. performed and analysed the in vivo rat experiments. G.L., T.K.J., J.T., J.H.P. and K.M.M. performed the work on the human birth cohort, and D.M.K., H.L., B.J. and K.M.M. prepared the manuscript.
Funding
The work was supported by the European Commission (EU-F7 grant # 212844 and 212502), the Villum Kann Rasmussen Foundation, the Novo Nordisk Foundation, Inserm (Institut National de la Santé et de la Recherche Médicale) and Ministère de l'Enseignement Supérieur et de la Recherche.
Acknowledgements
We thank P. Koopman for providing comments on the manuscript and J. Olsen for access to telephone interview data from the Danish National Birth Cohort.
Conflict of interest: The sponsors had no part in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors are solely responsible for the contents of the manuscript. The European Commission, the VRK Foundation, the Novo Nordisk Foundation and INSERM are not responsible for any use that might be made of data appearing therein. Therefore, we declare that we have no conflicts of interest.




Comments
To
The Editor
Human Reproduction
Respected Sir,
I have read with interest the article by Kristensen DM et al. (1). The authors have found that exposure to mild analgesics in intrauterine life is a risk factor for development of male reproductive disorders. However I have a few observations that I would like to enumerate.
Firstly, the authors failed to state whether or not the questionnaire used by them was pre-tested. If it had been pre-tested, the discrepancy observed between the response to computer-assisted telephone interview and the response to questionnaire might have been avoided.
Secondly, undescended testes may have familial occurrence and is also associated with multiple gestation (2). However, this does not seem to have been taken into consideration during the analysis.
Thirdly, the boys participating in the study were only examined at birth. 50% of cases of undescended testes at full-term birth may complete their descent within 1 month of birth (3). It would have been interesting to know if the differential observations regarding the descent of the testes persisted at one month interval also.
Thanking you,
With regards,
Debajyoti Datta
References:
1. Kristensen DM, Hass U, Lesne L, Lottrup G et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum. Reprod. 2010 : deq323v1- deq323. 2. Brouwers M, Feitz W, De Gier R, Zielhuis G, Roeleveld N. Endocrine Disruptors: Risk Factors For Undescended Testes. Journal of Pediatric Urology 2009; 5 (Supplement 1): S78. 3. Scorer CG. The descent of the testis. Arch Dis Child 1964;39:605-609.
Conflict of Interest:
None declared